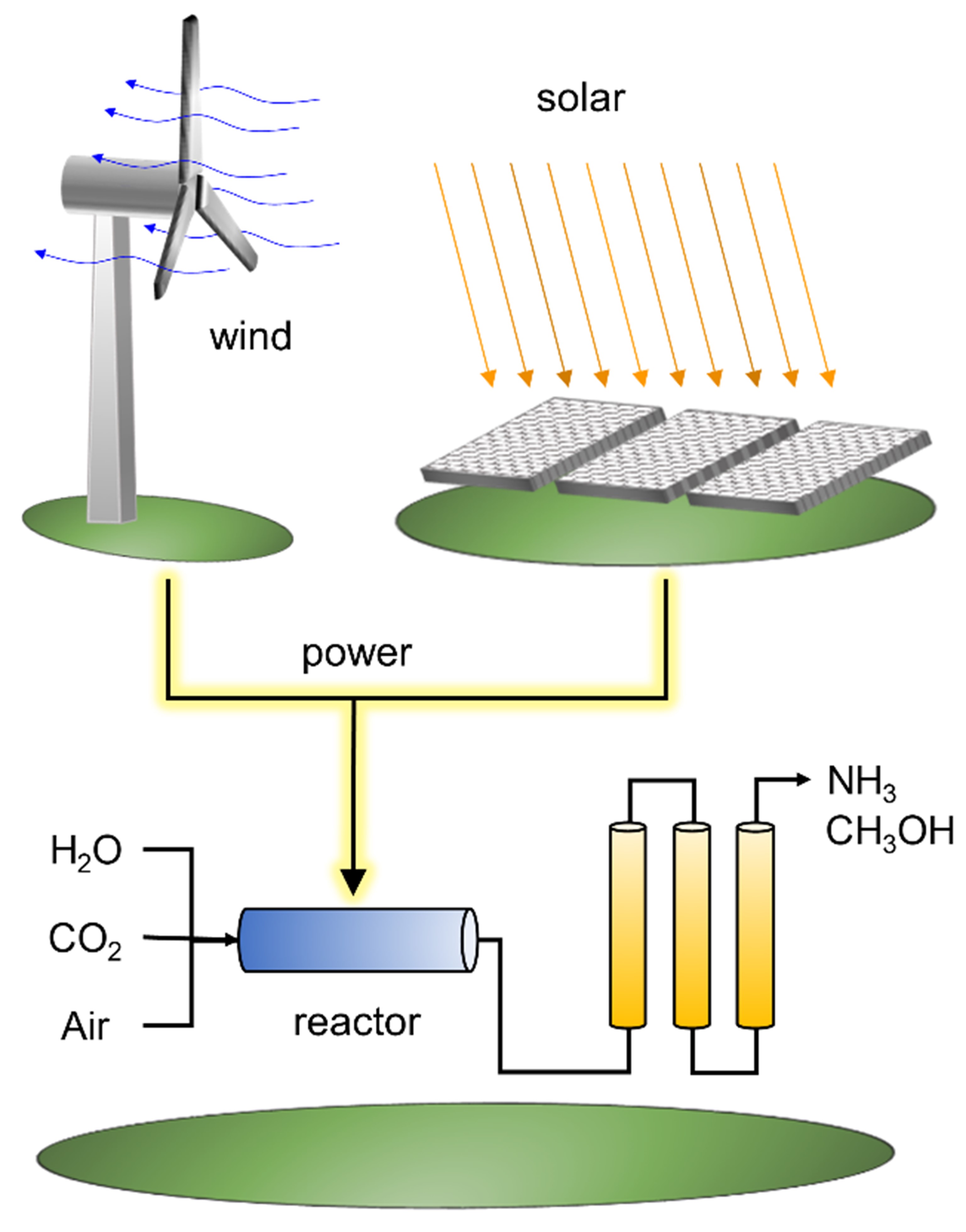
The emerging opportunity in the early 21st century is the utilization of low-cost renewable electricity. Large-scale implementation of wind turbines and photovoltaics are now providing electrical power competitive with fossil fuels absent concomitant emissions in CO2. These energy technologies provide a pathway to a carbon-free energy future, provided renewable power can be stored for times when we need it and transported to locations of high population density. The breakthrough technology opportunity of the next decade is to catalytically convert low-cost renewable power to carbon-free liquid fuels that can enable 100% implementation of wind turbines, solar photvoltaics, and other renewable power technologies.
Storage of renewable power as carbon-free or net-zero carbon fuels requires a dramatic improvement in the performance of catalysts that promote the chemical transformation of energy-storing molecules. Four key reactions determine our energy-managment future:
- Electrolysis of Water to H2: Water is available as a hydrogen source, but it must first be converted electrocatalytically from H2O(l) to H2(g) and O2(g).
- Ammonia Synthesis: Renewable H2(g) gas can be combined with N2(g) obtained from air to form ammonia, NH3, a small energy-dense molecule that can be condensed into a liquid.
- Reduction of CO2: Separation of carbon dioxide from the air provides a negative source of carbon that can be combined with H2(g) to form liquid methanol, CH3OH(l).
- Methanol Synthesis: Carbon monoxide prepared from reforming of waste materials (i.e., gasification) provides one-carbon feedstocks that can be reduced to liquids such as methanol.
While these reactions have been studied for a century, a new approach is required to advance catalytic conversion to achieve faster rates in smaller, more efficient catalytic reactors.
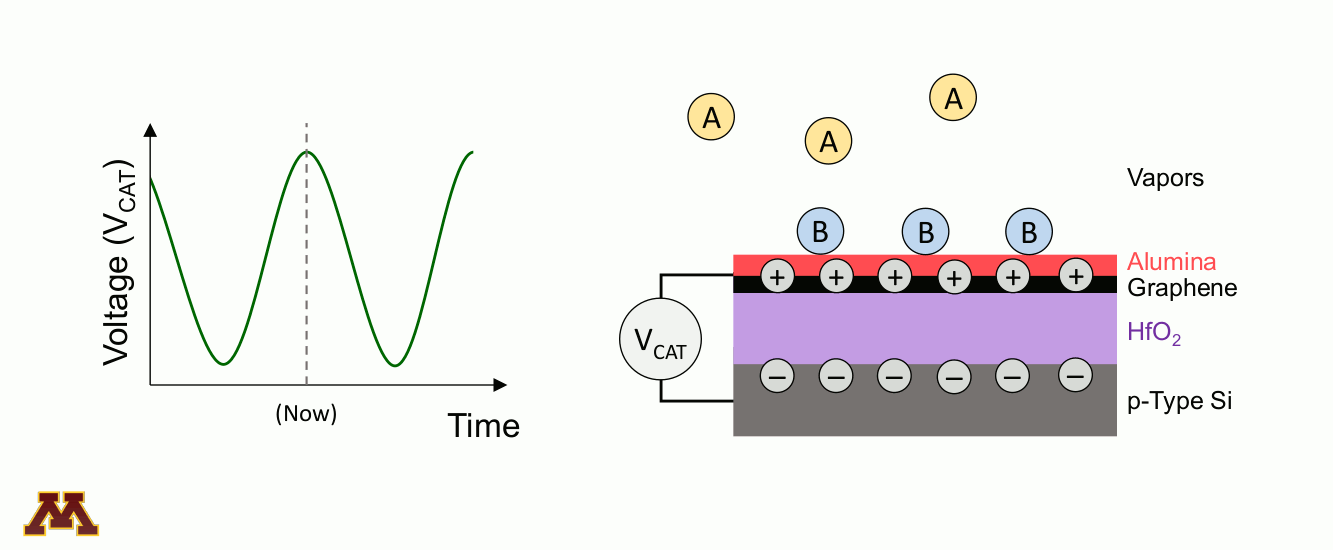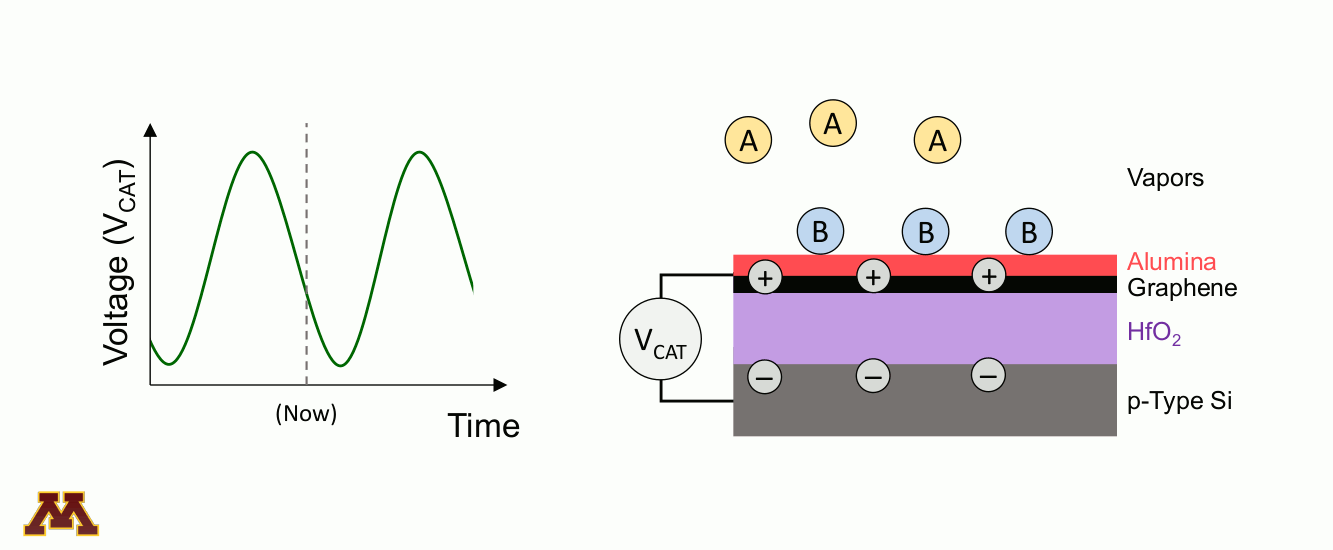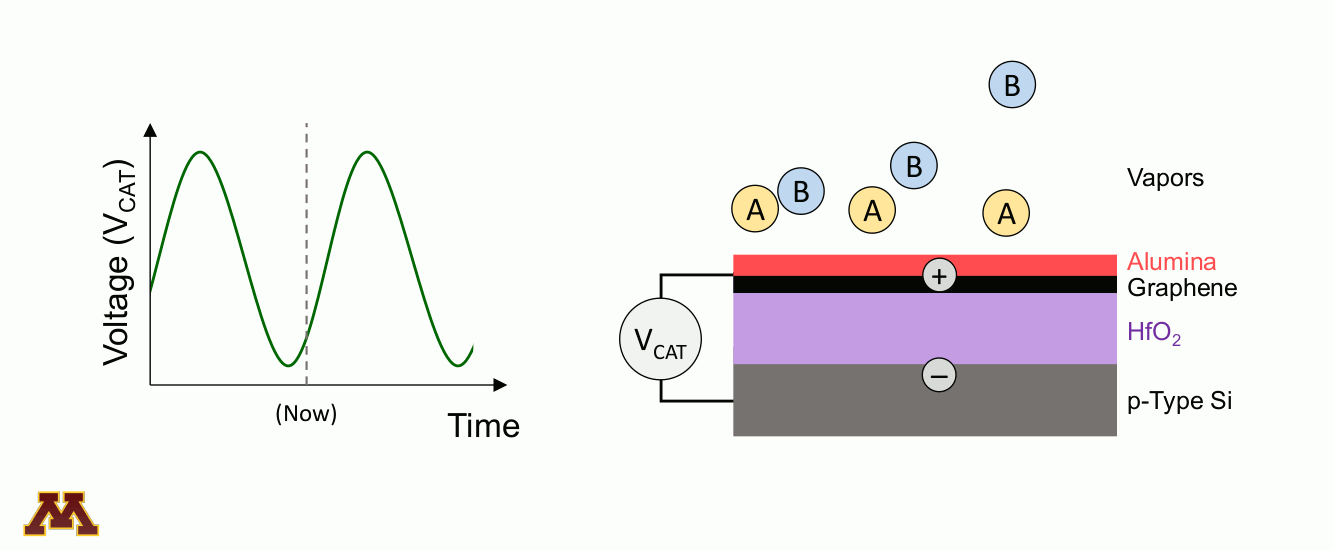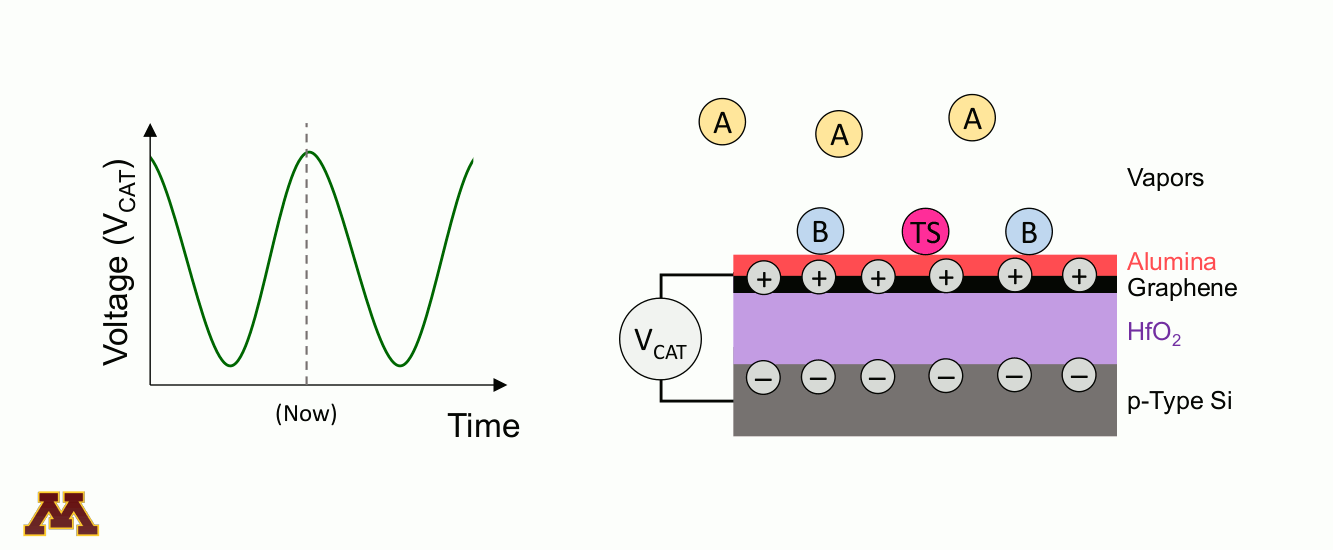
Catalytic reactions can be accelerated and controlled via a new approach of "programmable catalysts." Chemicals on a catalyst surface can be perturbed by pulsed light or by electronically manipulating the electron/hole density of the catalyst surface. Within a 'catalytic condenser' example below, an alumina/graphene active bilayer undergoes oscillating applied bias (VCAT), allowing it to shift its strength of acidity with time. Reaction occurs when electrons have been depleted, and desorption of products proceeds under the least acidic condition.
Within the Center for Programmable Energy Catalysis, research will focus on using dynamic light and oscillating catalytic condensers to control and optimize catalytic reactions critical to a low-carbon energy future. Programmable catalysts are designed, fabricated, and characterized for study in catalytic reactors, with optimization occuring through the modification of the applied light or electronic input to the catalytic surface. Identifying dynamic catalytic surfaces that accelerate targeted elementary reactions will provide entirely new capabilities for catalytic throughput, selectivity, and conversion, thereby enabling energy storage as carbon-free energy fuels.